In the chemistry industry, a packed distillation column (or fractionating column) is a kind of bed which aids in the separation process. when placed into the distillation column, different materials achieve different effects. The separation of compounds in this phase involves processes like:
- Absorption
- Distillation
- Stripping
- Fractioning
Distillation columns that are able to hold packing are usually an empty tube that can be filled with packing material, such as metal, glass, and various other materials.

Glass Raschig Rings – “Random” Packing
Packing a Column: Random vs. Structured
When packing a column, you can choose to have either a random packed column or utilize structured packing.
Randomly packed columns are created by randomly placing packing into the center of the distillation head. Structured packing, on the other hand, can be arranged or stacked in a set pattern.
Using packing offers the advantage of lower pressure drops across the fractionating column when compared to plates or trays. In some cases, this result can be beneficial when operating under vacuum.
Packed distillation columns allow gas to flow over the packing placed within the distillation head, while liquids tend to wet the surface of the packing material itself. This, in turn, allows mass transfer to take place. Mass transfer is the total movement of mass from one location to another, which includes the separation of chemicals in distillation columns.

An example of a connected packable distillation head
Different packing materials will have different surface areas and spacing between them. These variables will affect the performance of the distilling column. When distilling lower-boiling-point compounds, packing materials like heli-pack, glass raschig rings, and pro pak packing work well.
However, when distilling higher boiling point compounds, random packing tends to flood in some cases, due to uneven gas flow. This can hinder separation when compared to a more organized, structured packing.
The use of structured packing here allows a more even flow rate of these heavier compounds, which ends up spreading the gases evenly throughout the column. This reduces flooding, aids in separation, and increases flow rate.
Distillation Column: Packing Class & Definition
Random: Random packing material for distillation head columns are arbitrary in their placement. It does not matter how they are placed into the column, because you do not need/want to control the arrangement of the packing.
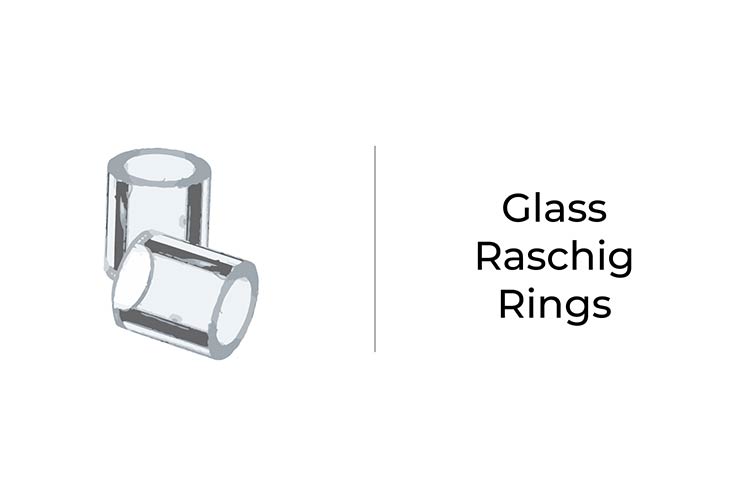
Raschig Rings: Usually small, circular or round pieces that can be glass, ceramic, or metal. They are considered random packing, as their small size and unique shape make them very difficult to organize inside the column.

Pro Pak: Are spherical pieces of stainless steel which have raised points on them. They create more surface area for the condensation of vapor. These are random packing and cannot be strategically placed in the distillation column.
Heli-pak: This packing is made of stainless steel wire, wrapped in a helical pattern, allowing for an increased surface area.

Structured: This packing is specially designed for absorption, and it is usually arranged symmetrically, forcing fluids to take certain pathways through the column. The slots or grooves increase surface area.
Flexipac: This type of structured packing has various, patterned angles and arrangements which change depending on the shape used. They help lower the pressure, aid in yield, and have reduced liquid hold-up.
Tower Packing: Packing of this sort contains a high ratio of surface area when compared to the volume that it takes up in the column. This style of packing is usually produced by knitting together metallic filaments, which are then crimped and woven into a spiral or simply folded and layered over itself.
Wire Gauze: Packing of this kind contains a wire gauze material and is designed for deep vacuum and low liquid rate applications. Due to its structure, it can provide an increased surface area, creating an enormously wettable surface. This structure allows excellent mass transfer to occur, specifically at low liquid rates.
Fractionation Variables
Fractional variable include, but are not limited to:
- Flow rate
- Size of packing
- Surface area
- Theoretical plates
- Vapor pressure
- Temperature differentials
When carrying out a distillation, reflux is an important process that takes place. The reflux process involves heating a liquid until it becomes a vapor. When this vapor comes into contact with the packing material, it condenses as it cools. Under optimal conditions, the vapor and liquid on each plate (or tray) is at an equilibrium, and the lighter boiling-point compounds can work their way further up the column to be fractionated. The heavier boiling point compounds, on the other hand, will condense and drip back down to be heated again.
This process is constantly repeated as the temperature rises, and fractions with different boiling points are pushed into the condenser. Processes such as these heavily contribute to how the fractionation of compounds takes place, and they directly relate to the quality of your final product.
Flooding
Having a correctly packed fractionating column will aid in fractionation tremendously, while having the wrong packing (or an over-packed column) can lead to flooding. Flooding stops proper fractionating from occurring, and it can possibly even contaminate your end product.
Flooding occurs when the trays get overloaded with excessive vapor due to overheating of the reaction flask or just heating it too quickly. This leads to a high vapor flow, which in turn forces liquid up the column. This liquid builds up and floods the column, stopping the proper fractionating process.
It is important to eliminate or reduce flooding to avoid contaminating your final product and be sure to never pack your column too tightly or put too much packing in your column.
Additional Types of Distillation Column Packing
As you can imagine, there are various other kinds of packing material out there, some structured and some not. All of them will subtly affect separation. You should perform research in your own lab to ensure that you are selecting the best kind of packing for maximum efficiency in your operation. Different goals require different types of packing to get there!
These and various other factors can all contribute to having an ideal distillation set up in your laboratory. Selecting the correct packing requires time and testing, but the results are worth it!
As a final note, you should also conduct thorough research on the specific compounds you are trying to distill/separate before you spend money on the wrong packing.
Having a correctly packed distillation column will help with your separation tremendously, while the wrong packing (or an over-packed column) can lead to reduced fractionation and possible contamination, due to the inability to properly fractionate.
Happy packing!